Paxalisib
Despite all efforts, the prognosis for patients with brain cancer has improved little in the past two decades. For glioblastoma, the most common and most aggressive form of brain cancer, average life expectancy from diagnosis is around 15 months, and less than 5% of patients are still alive after five years.
Developing New Therapies for Brain Cancer
Paxalisib (originally GDC-0084) was invented by Genentech, Inc (South San Francisco, CA), the most successful developer of new cancer medicines in the world. Kazia entered into a worldwide exclusive license agreement with Genentech in October 2016.
Well-Proven Mechanism of Action
Genentech designed paxalisib to inhibit PI3K, a critical control mechanism in growth and cell division, which is activated in many forms of cancer. PI3K is a very well-validated target for cancer drugs, with five FDA-approved therapies in the class: Zydelig® (idelalisib), Aliqopa® (copanlisib), Copiktra® (duvelisib), Piqray ® (alpelisib), and Ukoniq® (umbralasib). PI3K is a very attractive target for glioblastoma because more than 85% of patients have activation of this pathway.
The distinguishing feature of paxalisib is its ability to cross the blood-brain barrier (BBB). Ordinarily, the BBB prevents many drugs from reaching brain tissue, and is a challenge in the treatment of any disease in the central nervous system. Paxalisib has been designed to cross the BBB, and a wealth of experimental data shows that it does so very successfully. This feature of paxalisib is almost unique in this class of medicines and differentiates it from the approved products in the PI3K inhibitor class.
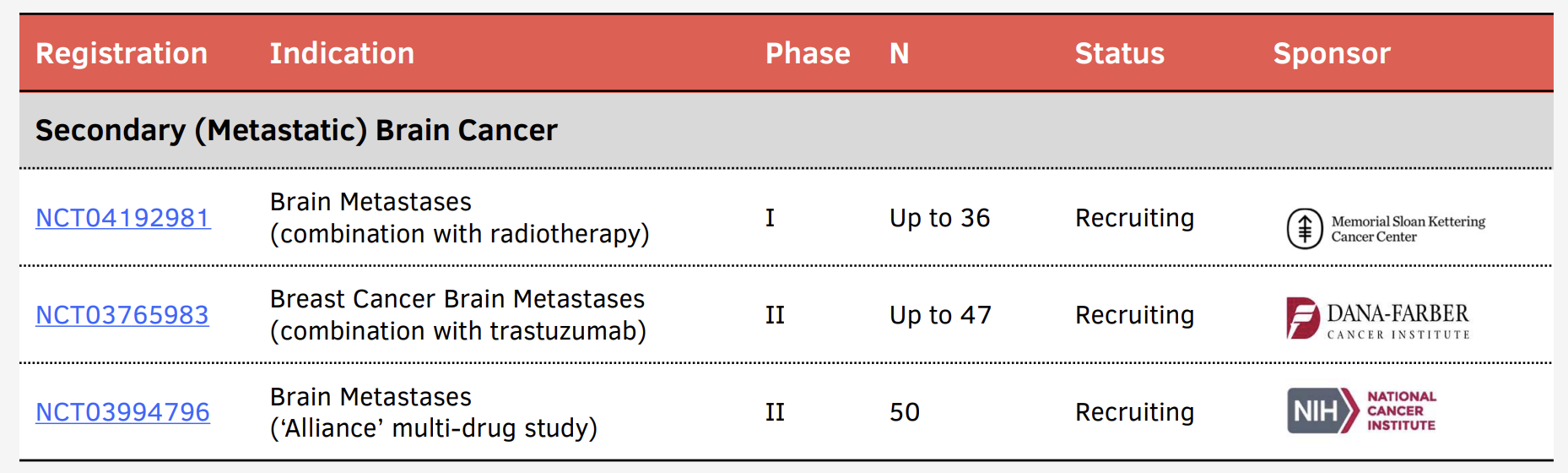
Broad Development Program
The lead indication for paxalisib is glioblastoma. The drug candidate is currently in an adaptive phase III study for potential registration called GBM AGILE. This study is sponsored by the Global Coalition for Adaptive Research, a US-based not-for-profit organisation, and uses cutting-edge statistical techniques to accelerate the development of new therapies.In this study, Paxalisib is being evaluated in patients with newly-diagnosed glioblastoma who have a genetic marker called an unmethylated MGMT promotoras well as relapsed glioblastoma.
In addition, more than half-a-dozen investigator-initiated studies are underway in other forms of adult and pediatric brain cancer or brain metastases at leading research hospitals across the United States. These studies are designed and largely funded by the institutions that run them, with input and support from Kazia. Potentially, the data they generate may provide a path to substantially expand the commercial use of paxalisib.

Substantial Commercial Opportunity
Glioblastoma affects approximately 130,000 patients per annum worldwide. It is conservatively estimated to represent a US$ 1.5 billion annual commercial market. If paxalisib can also be approved for other forms of brain cancer, that is expected to create additional opportunity for the product.

